Categories
Change Password!
Reset Password!


Bifidobacterium adolescentis PRL2019 improves symptom remission and normalizes bowel habits in children with IBS, particularly in those with the constipation-predominant subtype.
According to the findings of a randomized controlled trial, the probiotic strain Bifidobacterium adolescentis (B. adolescentis) PRL2019 markedly alleviates the symptoms of irritable bowel syndrome (IBS) in children—marking a promising advancement in gut microbiota-targeted therapy.
Emerging evidence underscores the fundamental role of the gut microbiota in gastrointestinal inflammation and immune modulation. In IBS, imbalances in microbial composition may disrupt neurotransmitter expression—particularly γ-aminobutyric acid (GABA), the central nervous system (CNS) chief inhibitory neurotransmitter. GABA levels are often reduced in IBS patients, contributing to altered gut sensory-motor function and symptom onset.
B. adolescentis PRL2019, a strain known to naturally produce GABA, has illustrated immunomodulatory potential. Hence, this study sought to evaluate its clinical efficacy in children diagnosed with IBS using Rome IV criteria. In total, 72 children (mean age 12.2 ± 1.8 years; 30 males) were enrolled and randomly assigned to get either one daily stick of B. adolescentis PRL2019, delivering 20 × 10⁹ colony-forming units, or an identical placebo for 12 weeks. Participants were evenly split into two groups (n=36 each), with no significant differences in baseline demographics, IBS subtype distribution, or symptom severity.
Symptom assessments were conducted at 4-week intervals using validated scales: Life Interference Score (LIS), Pain Frequency Score (PFS), Pain Intensity Score (PIS), and the IBS Symptom Severity Scale (IBS-SSS). Bowel habits were evaluated via the Bristol Stool Chart (BSC). By week 12, the probiotic group exhibited higher complete symptom remission when compared to the placebo group (odds ratio 0.216), as shown in Table 1:
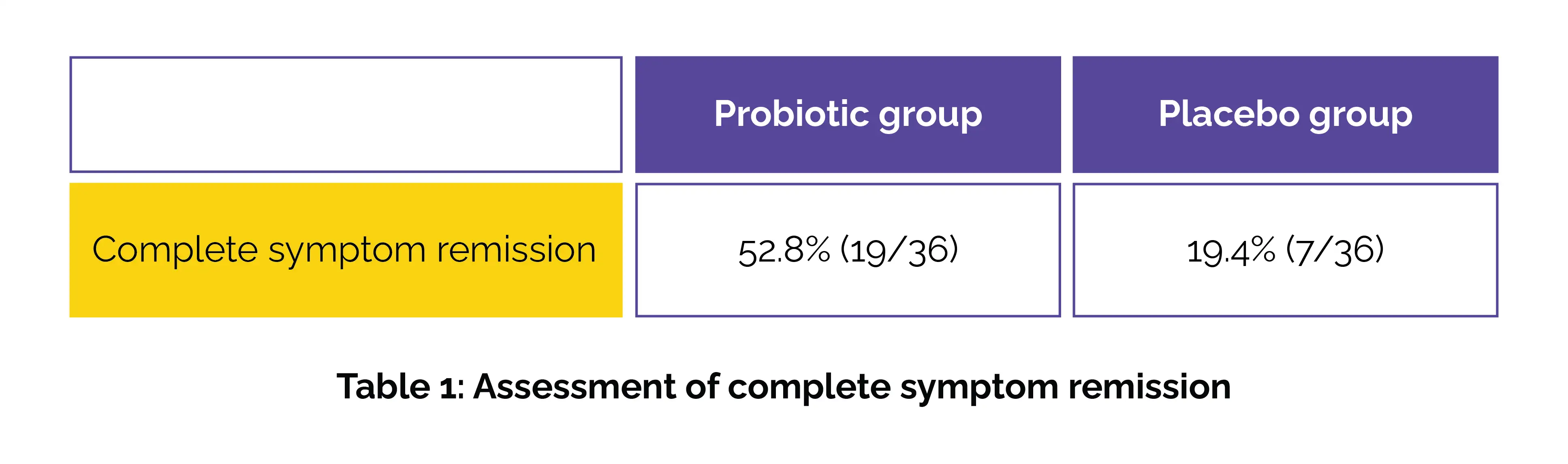
While both groups showed overall symptom improvement, intergroup analysis revealed remarkably greater reductions in the B. adolescentis group in IBS-SSS, PIS, PFS, and LIS. Moreover, stool normalization (types 3–4 on the BSC) enhanced markedly in the probiotic group—from 25% at baseline to 58.3% post-treatment. Among children with IBS-constipation subtype, the proportion of normal stools rose from 19.4% to 44.5%, highlighting the probiotic's specific benefit for this subgroup.
This study provides robust evidence that B. adolescentis PRL2019 is effective in easing symptom severity, frequency, and abnormal bowel habits in children with IBS—particularly for those with constipation-dominant presentations. By restoring GABA activity and modulating gut-brain signaling, this targeted probiotic offers a safe and non-invasive option for pediatric IBS care.
Microorganisms
Bifidobacterium adolescentis PRL2019 in Pediatric Irritable Bowel Syndrome: A Multicentric, Randomized, Double-Blind, Placebo-Controlled Trial
Valentina Giorgio et al.
Comments (0)