Categories
Change Password!
Reset Password!


Gepotidacin is an effective and safe oral therapy for uncomplicated urinary tract infections, showing comparable efficacy to nitrofurantoin and added benefit against resistant strains.
In a study published in “The Lancet”, oral gepotidacin, a new antibiotic that targets bacterial DNA, was safe and well-tolerated by adolescent and adult females with uncomplicated urinary tract infections (UTIs).
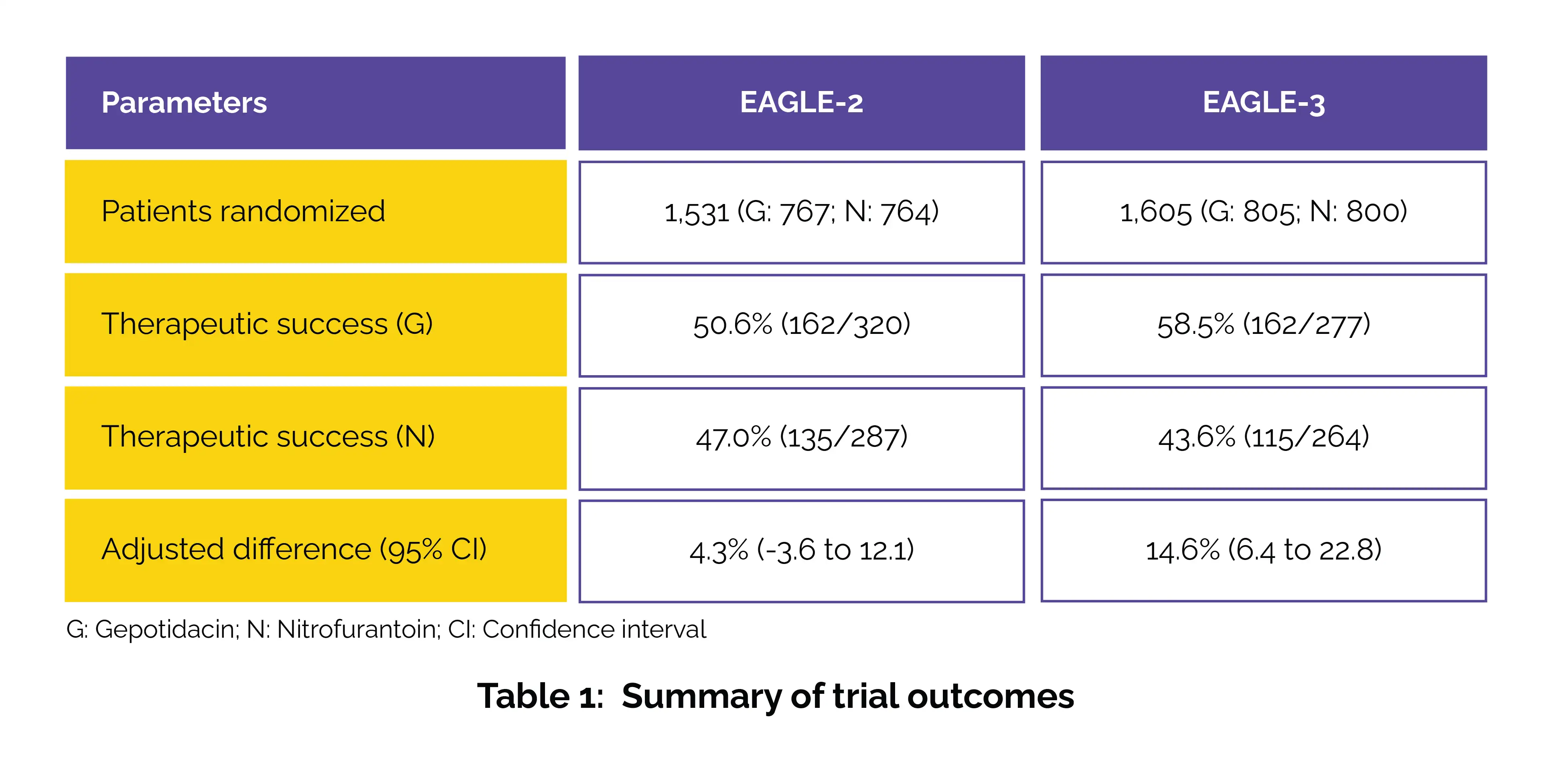
Researchers ran two large, multicentre, phase 3 trials (EAGLE-2 and EAGLE-3) across 219 sites, randomizing non-pregnant female patients (aged 12 years or older) with uncomplicated UTIs to receive either oral gepotidacin (1500 mg twice daily) or nitrofurantoin (100 mg twice daily) for 5 days. Participants were stratified by age and history of recurrent UTIs, with investigators, patients, and sponsors blinded to treatment allocation.
The primary endpoint of the trials was to check the treatment response at the test-of-cure visit, which took place 10–13 days after starting therapy. This analysis included patients who were randomly assigned, had nitrofurantoin-sensitive bacteria at baseline (≥100,000 colony- forming units [CFU]/mL), and received at least one dose of the study drug. Treatment success was defined as complete resolution of UTI symptoms and a reduction in bacterial counts to below 1,000 CFU/mL, without the need for additional antibiotics.
For safety, all participants who were randomized and took at least one dose of the treatment were monitored for side effects. In terms of outcomes, gepotidacin proved to be non-inferior to nitrofurantoin in both studies, and in one trial it also demonstrated superiority, as shown in Table 1:
In terms of safety, adverse events were generally mild-to-moderate. Diarrhea was the most common side effect reported with gepotidacin (14% in EAGLE-2 and 18% in EAGLE-3), while nausea was most frequently observed with nitrofurantoin (around 4% in both studies). Importantly, no life-threatening or fatal adverse events were reported.
Researchers conclude that gepotidacin represents a promising oral treatment option for UTIs, particularly in the context of rising antibiotic resistance. With its novel mechanism and activity against key drug-resistant strains, this first-in-class triazaacenaphthylene antibiotic could play a pivotal role in addressing the global need for new antimicrobial therapies.
The Lancet
Oral gepotidacin versus nitrofurantoin in patients with uncomplicated urinary tract infection (EAGLE-2 and EAGLE-3): two randomised, controlled, double-blind, double-dummy, phase 3, non-inferiority trials
Florian Wagenlehner et al.
Comments (0)