Categories
Change Password!
Reset Password!


Oral linzagolix, with or without hormonal add-back therapy, maintains a significant reduction in heavy menstrual bleeding through 52 weeks.
A new analysis from the PRIMROSE 1 and 2 phase 3 trials confirms that oral linzagolix, administered once daily at 100 mg or 200 mg—with or without hormonal add-back therapy (ABT)—effectively maintains its benefits in decreasing heavy menstrual bleeding in women with symptomatic uterine fibroids for up to 52 weeks.
The double-blind, randomized, placebo-controlled trials enrolled 1,012 women (aged 18 years or above) across 189 clinical sites in the United States and Europe. All the volunteers had ultrasound-confirmed fibroids and experienced menstrual blood loss exceeding 80 ml per cycle for at least two cycles. Subjects were randomly assigned to get placebo or one of four linzagolix regimens: 100 mg alone, 100 mg with ABT (1 mg estradiol and 0.5 mg norethisterone acetate), 200 mg alone (with ABT starting at week 24), or 200 mg with ABT. Researchers assessed efficacy and safety outcomes at week 52, and symptom recurrence during a 12-week withdrawal phase, with bone mineral density (BMD) monitored through week 76.
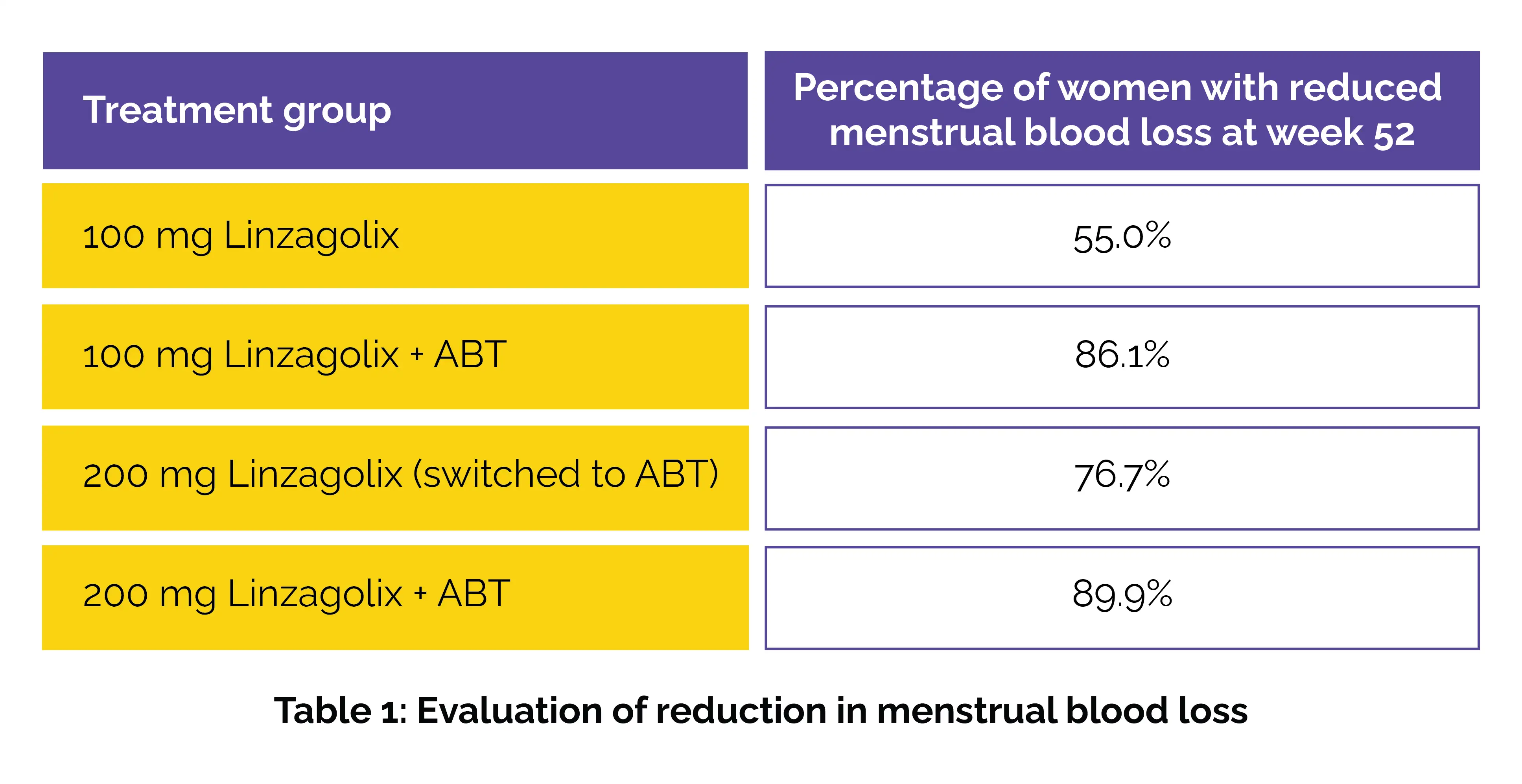
At the 52-week mark, linzagolix continued to show prominent reductions in menstrual blood loss when compared to baseline across all active treatment groups. Specifically, 55.0% of patients in the 100 mg group, 86.1% in the 100 mg + ABT group, 76.7% in the 200 mg group (switched to ABT), and 89.9% in the 200 mg + ABT group attained the primary endpoint—less than 80 ml of blood loss and more than 50% reduction from baseline.
Among those who achieved amenorrhoea by week 52 with linzagolix treatment, 88.8% experienced a return of bleeding—often heavy—between weeks 52 and 64. The improvements in bleeding and pain translated to better quality of life for the patients. Hot flushes were the most commonly reported side effect up to week 52 but typically resolved after treatment discontinuation.
Importantly, BMD remained stable across all treatment arms, with patients initially on 200 mg monotherapy experiencing BMD recovery after ABT initiation at week 24. However, during the withdrawal period, 88.8% of women who had attained amenorrhea by week 52 reported a return of bleeding—often heavy—by week 64, highlighting the likelihood of symptom recurrence following cessation. These findings underscore linzagolix’s potential as a long-term treatment option for women battling uterine fibroids.
Thus, the 100 mg dose without ABT offers a non-hormonal approach for those unable or unwilling to use ABT, while higher doses with ABT provide enhanced bleeding control with protection against bone loss and vasomotor symptoms. The rapid symptom return after stopping therapy supports the consideration of ongoing treatment for sustained relief.
Fertility and Sterility
Linzagolix with and without hormonal add-back therapy for symptomatic uterine fibroids: PRIMROSE 1 & 2 long-term extension and withdrawal study
Jacques Donnez et al.
Comments (0)