Categories
Change Password!
Reset Password!


Tegoprazan, a novel potassium-competitive acid blocker (P-CAB), has rapid and potent acid-suppressive properties.
Tegoprazan-based 14-day triple therapy is as effective as lansoprazole-based 14 day triple therapy for eradicating H. pylori.
Tegoprazan, a novel potassium-competitive acid blocker (P-CAB), has rapid and potent acid-suppressive properties. This study sought to compare the effectiveness of tegoprazan-based versus lansoprazole-based triple therapy regimens for Helicobacter pylori (H. pylori) management.
Patients diagnosed with H. pylori infection who were prescribed either a 14-day tegoprazan- or lansoprazole-based triple therapy as initial therapy were retrospectively assessed. Patient records were reviewed to analyze H. pylori elimination success and adherence rates for each regimen.
Among 670 patients, 64 received tegoprazan-based triple therapy and 295 received lansoprazole-based triple therapy as their first-line therapy. Treatment adherence was comparable between the groups. The H. pylori elimination rates in the intent-to-treat and the per-protocol analysis is depicted in Table 1:
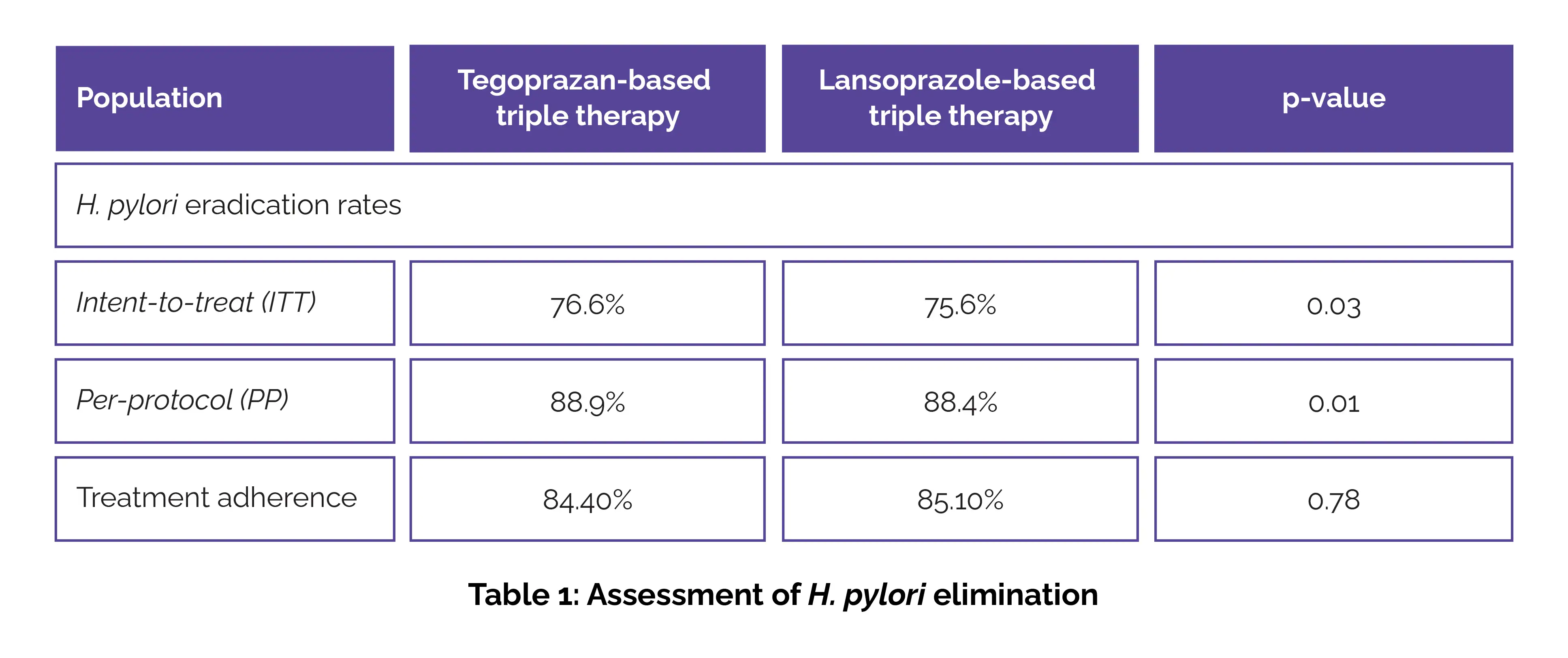
Non-inferiority was confirmed in both analyses (p=0.03 and p=0.01).
For the eradication of H. pylori, 14-day tegoprazan-based triple therapy was non-inferior to lansoprazole-based therapy, supporting its use as a promising first-line option.
Korean Journal of Helicobacter and Upper Gastrointestinal Research
Comparison of Tegoprazan- and Lansoprazole-Based Fourteen-Day Triple Therapies as First-Line Treatments for Helicobacter pylori Eradication
Seokin Kang et al.
Comments (0)