Categories
Change Password!
Reset Password!


In patients with erosive GERD, tegoprazan shows non-inferior efficacy to esomeprazole with 99.1% healing rate at 8 weeks.
A phase 3 clinical trial has shown that tegoprazan, a next-generation potassium-competitive acid blocker (P-CAB), provides rapid and sustained acid suppression with healing outcomes comparable to esomeprazole in patients with erosive gastroesophageal reflux disease (eGERD).
This randomized controlled study was carried out across 3 countries—India, Russia, and South Africa. A total of 255 patients (between 18 and 65 years of age) with endoscopically validated eGERD classified as Los Angeles grades A–D, were enrolled after a 14-day screening period. Volunteers were randomized in a 1:1 ratio to get once-daily tegoprazan 50 mg or esomeprazole 40 mg for 8 weeks. By week 8, the cumulative endoscopic healing rate was slightly higher for the tegoprazan group when compared to the esomeprazole group (Table 1):
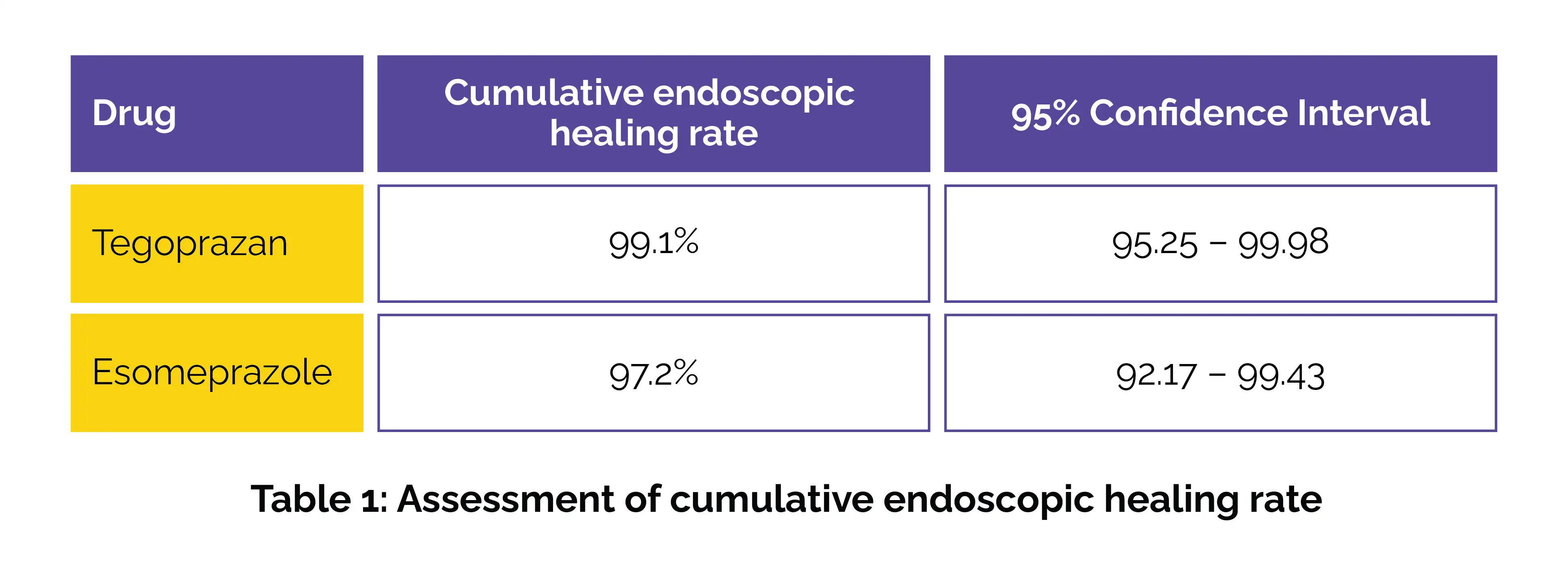
These findings confirmed tegoprazan's non-inferiority with high statistical confidence (p < 0.0001). Importantly, tegoprazan’s rapid mechanism of action—achieving and maintaining an intragastric pH above 4 following both single and multiple doses—offers patients with early relief and sustained mucosal healing. Tegoprazan was well-tolerated, with a safety profile similar to esomeprazole. In both the arms, treatment-emergent adverse events (TEAEs) occurred at same rates.
The most common TEAEs, witnessed in at least 1% of subjects were abdominal pain, headache, diarrhea, and nausea. No unexpected safety signals were observed throughout the trial, reinforcing tegoprazan’s suitability for long-term clinical use. These findings position tegoprazan as a safe, effective, and well-tolerated option for erosive GERD care, with healing outcomes on par with esomeprazole and the added advantage of rapid acid suppression.
Japanese Journal of Gastroenterology
Efficacy and Safety of Tegoprazan in Patients with Erosive Gastroesophageal Reflux Disease- A Multicountry, Prospective, Randomized, Double-Blind, Active-Controlled, Parallel-Group Study
Piyush Agarwal et al.
Comments (0)