Categories
Change Password!
Reset Password!


Type 2 diabetes mellitus (T2DM) is a surging global health challenge, driven by insulin resistance and progressive β-cell impairment.
GLP-1RAs improve blood sugar control in T2DM, with tirzepatide being most effective for weight loss in obesity. In normal-weight patients, semaglutide and liraglutide are effective, with liraglutide offering a safer profile for reducing the risk of hypoglycemia.
Type 2 diabetes mellitus (T2DM) is a surging global health challenge, driven by insulin resistance and progressive β-cell impairment. The International Diabetes Federation predicts that over 780 million people worldwide will be affected by 2045, emphasizing the urgent need for effective therapeutic strategies. Glucagon-like peptide-1 (GLP-1), an intestinal hormone released after food intake, enhances insulin secretion and supports β-cell function to maintain glucose balance. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) exploit this pathway to lower blood glucose by stimulating insulin release and suppressing glucagon.
According to the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD), GLP-1RAs can be used both as add-on therapy to lifestyle modification and as an alternative when metformin monotherapy does not provide sufficient glycemic control. These agents are categorized by their duration of action into long-acting agents—such as semaglutide, tirzepatide, liraglutide, dulaglutide, albiglutide, and once-weekly exenatide (EQW)—and short-acting agents like twice-daily exenatide (EBID) and lixisenatide.
Evidences from clinical trials indicate that GLP-1RAs not only improve glycemic control but also aid weight reduction more successfully than traditional antidiabetic therapies. However, direct comparisons among these agents and against traditional antidiabetic drugs remain limited. Clarifying their relative efficacy and safety is essential for precision therapy, enabling clinicians to tailor treatments to patient profiles and optimize outcomes in T2DM.
Objective
Researchers evaluated and compared the clinical efficacy and safety of GLP-1 RAs, while also assessing their performance against traditional antidiabetic therapies through the largest network meta-analysis (NMA) to date.
The study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses (PRISMA-NMA) framework to ensure methodological rigor and transparency.
Literature search
A comprehensive systematic search was conducted across PubMed, Cochrane Library, Embase, Web of Science, and Chinese databases including China National Knowledge Infrastructure (CNKI), WangFang, and VIP (China Science and Technology Journal Database) up to October 2024.
Database searches were performed via a combination of terms such as: “Glucagon-Like Peptide-1 Receptor Agonists,” “Exenatide,” “Semaglutide,” “Dulaglutide,” “Liraglutide,” “Albiglutide,” “Tirzepatide,” “Lixisenatide,” “Type 2 Diabetes Mellitus,” and “Randomized Controlled Trials.”
Moreover, reference lists of all the eligible studies were manually screened to explore more suitable studies. Two independent reviewers executed the literature search and study selection, and any discrepancies were cleared via discussion and consensus.
Inclusion criteria
Studies were deemed eligible if they fulfilled the below conditions:
Two researchers independently screened and examined studies; disagreements were solved by consensus.
Exclusion criteria
Search and study characteristics
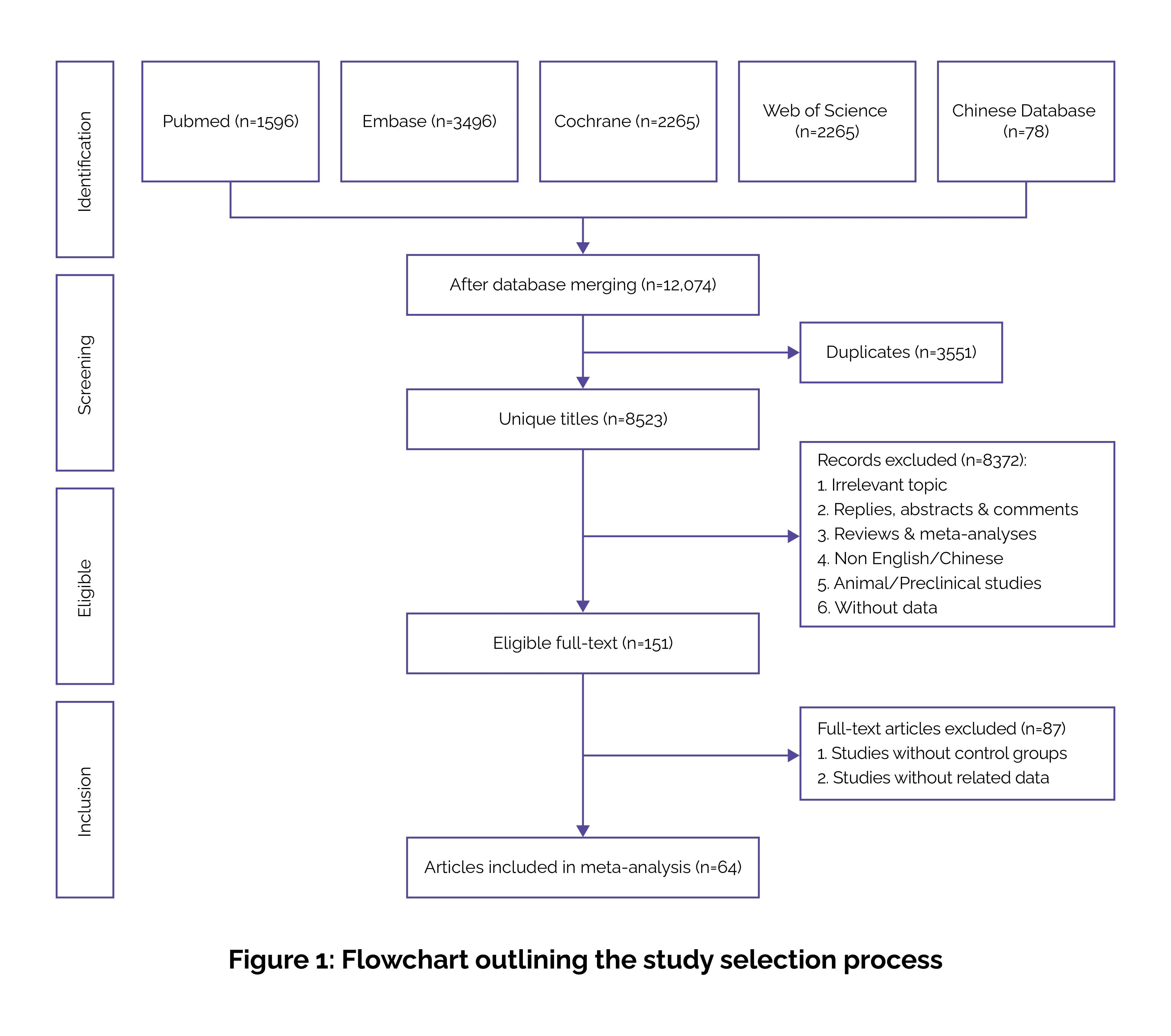
Data extraction and handling
Quality assessment and statistical analysis
This ensured a rigorous, transparent, and comprehensive comparison of GLP-1RAs and traditional antidiabetic therapies, supporting clinically meaningful conclusions.
(a) HbA1c (53 trials, 21,486 patients)
SUCRA rankings confirmed tirzepatide as the top performer, followed by semaglutide and liraglutide.
(b) FPG (41 trials, 17,621 patients)
(c) Secondary outcomes
(d) Safety
(e) Consistency & reliability
By pooling evidence from multiple RCTs, this NMA provides a clear, evidence-based guide for clinicians, helping them personalize T2DM care and maximize patient outcomes.
Semaglutide stands out with two formulations: injectable and oral. The injectable version has a fatty acid side chain that shields it from enzymatic breakdown, giving it a half-life of 1 week. The oral tablet uses a special absorption enhancer for preventing degradation in the stomach. Despite these differences, both forms are equally efficient, though the oral version may boost patient adherence owing to its convenience.
For analysis, both formulations were treated as a single intervention. The review included 64 trials with over 25,500 volunteers. Long-acting GLP-1RAs consistently outperformed short-acting ones in minimizing HbA1c and FPG. Among them, tirzepatide, semaglutide, and liraglutide delivered the strongest improvements compared with other GLP-1RAs, traditional antidiabetic drugs, and placebo, confirming their superior glycemic benefits. When it comes to weight management, tirzepatide, semaglutide, EBID, and liraglutide led to considerable weight loss versus placebo and standard treatments.
Notably, tirzepatide and semaglutide were more potent than other GLP-1RAs. Tirzepatide, a dual glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 RA approved in 2022, combines enhanced blood sugar control with marked weight reduction, making it particularly suitable for obesity management. Liraglutide, on the other hand, may be ideal for those who do not require weight loss, such as older adults or those at risk of sarcopenia. Regarding safety, semaglutide, dulaglutide, liraglutide, and tirzepatide were related to a higher incidence of gastrointestinal adverse effects.
EBID and semaglutide escalated the risk of hypoglycemia, whereas liraglutide and lixisenatide lowered it compared to traditional therapies. No major differences were noted among GLP-1RAs themselves. These findings suggest careful selection based on individual patient profiles: liraglutide may be safer for those prone to gastrointestinal issues or hypoglycemia, while tirzepatide is preferable for those needing weight reduction. In short, the choice of a GLP-1RA should consider blood sugar control, weight impact, risk of hypoglycemia, and gastrointestinal tolerance.
This study had several limitations.
Choosing the appropriate GLP-1RA involves balancing therapeutic benefits, potential risks, and patient-centered goals. Among GLP-1RAs, tirzepatide shows the strongest reductions in HbA1c, fasting glucose, and body weight, making it most suitable for T2DM patients with obesity. Semaglutide and liraglutide remain effective for normal-weight individuals, though semaglutide carries a higher hypoglycemia risk, while liraglutide is safer. Gastrointestinal adverse effects are common across all GLP-1RAs, whereas impacts on blood pressure and lipid levels are minimal.
Scientific Reports
Efficacy and safety of GLP-1 agonists in the treatment of T2DM: A systematic review and network meta-analysis
Xiaoyu Ren et al.
Comments (0)