Categories
Change Password!
Reset Password!


Allergic conjunctivitis is the most common ocular allergy, triggered by environmental allergens like pet dander, dust mites, and pollen.Allergic conjunctivitis is the most common ocular allergy, triggered by environmental allergens like pet dander, dust mites, and pollen.
NAAGA outperforms azelastine in alleviating both symptoms and clinical markers of tear film dysfunction in allergic conjunctivitis, owing to its dual anti-inflammatory and antiallergic benefits.
Allergic conjunctivitis is the most common ocular allergy, triggered by environmental allergens like pet dander, dust mites, and pollen. It arouses characteristic symptoms like redness, itching, tearing, and conjunctival inflammation, and in more severe cases, may also involve the cornea. The ailment results from immune hypersensitivity responses in predisposed individuals, making clinical understanding of its mechanisms essential for effective care.
A substantial challenge in tackling allergic conjunctivitis is its frequent overlap with dry eye disease (DED), particularly during allergy-prone seasons like spring. Both ailments are influenced by similar environmental triggers—like climate and pollution—and share core pathophysiological features encompassing inflammation and tear film dysfunction. The extended inflammatory reaction associated with ocular allergies leads to an increase in inflammatory mediators within the tear film. This elevation contributes to damage of the nerve fibers and the epithelial cells of both the cornea and conjunctiva.
This worsens tear film instability, a hallmark of DED. In turn, tear film dysfunction and poor tear clearance in DED prolong the retention of allergens and pro-inflammatory substances on the ocular surface, intensifying allergic symptoms. This bidirectional interaction creates a vicious cycle that complicates diagnosis and treatment. Given this overlap, optimal treatment in atopic individuals must address both allergic inflammation and DED-linked tear instability. Conventional interventions for ocular allergy encompass antihistamines—such as emedastine, azelastine, and epinastine—which block histamine H1 receptors, and mast cell stabilizers—like sodium cromoglycate and N-acetyl-aspartyl-glutamate (NAAGA)—which hinder mast cell degranulation and release of histamine.
NAAGA, also known as spaglumic acid, has emerged as a particularly promising agent due to its dual antiallergic and anti-inflammatory properties. Beyond mast cell stabilization, NAAGA also suppresses the activation of inflammatory cells, the complement cascade, and leukotriene secretion. Previous research by Brignole-Baudouin and colleagues showed that NAAGA downregulates human leukocyte antigen – DR isotype (HLA-DR), a marker of conjunctival inflammation, supporting its utility in DED. Additional findings by Shin et al. illustrated NAAGA’s effectiveness in managing DED, with faster symptom relief and fewer side effects than cyclosporine A, which is often linked with delayed onset and tolerability issues.
Importantly, NAAGA has shown promise as a standalone intervention for moderate allergic conjunctivitis. Lazreg et al. found it to be well-tolerated and beneficial without requiring corticosteroids, offering a safer long-term option. Comparatively, azelastine, a second-generation antihistamine, remains widely used owing to its potent H1 receptor antagonism, mast cell-stabilizing effects, and low affinity for cholinergic receptors—making it beneficial in minimizing allergic inflammation with minimal systemic side effects.
Despite their different mechanisms, both NAAGA and azelastine are key players in the current therapeutic landscape. However, NAAGA’s ability to target both the allergic and dry eye components offers an integrated approach that could enhance outcomes in those with overlapping symptoms.
Objective
This randomized controlled trial sought to compare the effectiveness of NAAGA versus azelastine hydrochloride in ameliorating symptoms and clinical indicators of tear film disruption in individuals with allergic conjunctivitis.
Study design
In this prospective, single-blind study, volunteers were randomly assigned utilizing a computer-generated sequence with allocation concealment through sealed opaque envelopes. Outcome assessment was performed by a blinded investigator, while another investigator handled enrollment, randomization, and final group allocation. Written informed consent was procured from all the volunteers prior to their enrollment.
Inclusion criteria
Exclusion criteria
Excluded patients included those with:
Interventions
The enrolled subjects were randomly allocated to get either 4.9% NAAGA administered 4 times daily, or 0.05% azelastine hydrochloride administered twice daily. Both therapies were given as single-dose formulations over a 4-week period. Dry eye evaluations were executed at baseline (week 0), week 2, and week 4, using a standardized sequence of tests to avoid interference among procedures:
In addition to clinical assessments, the enrolled subjects completed a weekly self-assessment questionnaire throughout the study period to monitor subjective symptoms of ocular discomfort, starting from day 0 (study enrollment).
Data and statistical analysis
A post-hoc power analysis confirmed the adequacy of the study’s sample size. Notably, with 80 people in Group A and 54 in Group B, and using an effect size of 2.13 for the between-group ΔOSDI comparison at a significance level of 0.05, the calculated statistical power exceeded 0.99. Furthermore, within-group pairwise comparisons of OSDI scores across week 0, week 2, and week 4 showed a minimum power of 0.98, indicating a highly sufficient sample size to detect meaningful changes.
Data analysis began with descriptive statistics, and normality was evaluated via the Shapiro–Wilk test. Depending on the data distribution:
A p-value of less than 0.05 was deemed statistically significant. All statistical analyses were executed using SPSS software.
Study outcomes
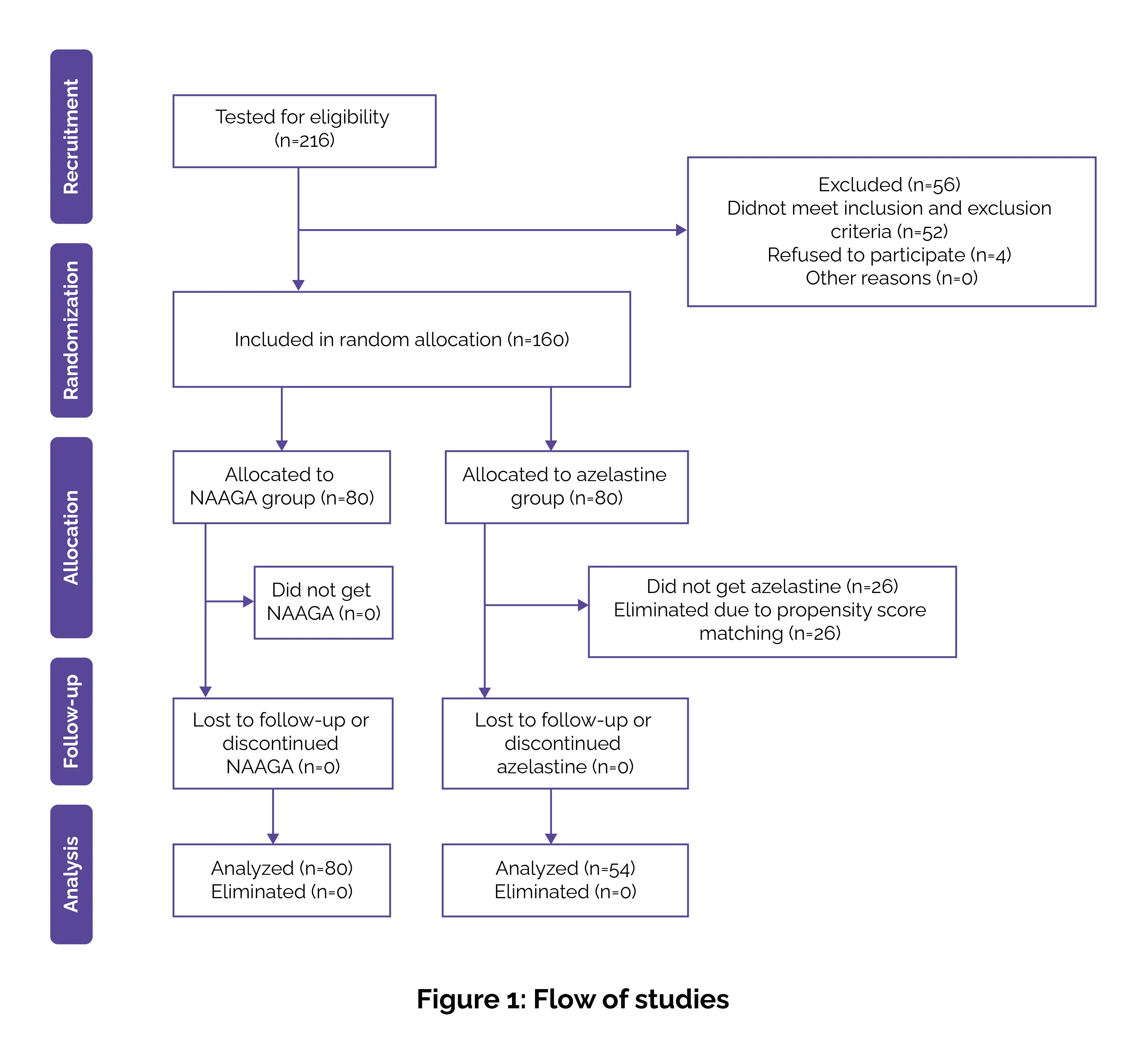
Key findings
Both NAAGA and azelastine led to remarkable improvements in all measured parameters over 4 weeks, with NAAGA demonstrating superior efficacy:
In this trial, both NAAGA and azelastine markedly improved the symptoms of DED and allergic conjunctivitis in patients with an atopic background. However, NAAGA was found to be more beneficial in ameliorating tear film parameters, with greater improvements in OSDI scores, tear osmolarity, and TBUT. These findings suggest that NAAGA may yield superior anti-inflammatory benefits and tear film stabilization than azelastine.
DED and allergic conjunctivitis share overlapping pathophysiology, often sparked off by environmental factors such as seasonal allergens, humidity, and temperature changes. These external stimuli elicit immune responses that impair tear film stability and the ocular surface, creating a cycle of inflammation. Studies have identified shared biomarkers—such as interleukins (IL)-1α, IL-1β, IL-17, tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ)—indicating that both ailments involve common inflammatory pathways.
Azelastine, a selective second-generation H1 receptor antagonist, offers prompt relief from allergic conjunctivitis symptoms by blocking histamine receptors and stabilizing mast cells. It also suppresses inflammatory mediators like leukotrienes, prostaglandins, and platelet-activating factor (PAF), helping monitor both early and late allergic responses. Although its anticholinergic activity is low, azelastine may still minimize tear production, potentially aggravating DED symptoms in a few patients
In contrast, NAAGA functions as a mast cell stabilizer with broader upstream anti-inflammatory effects. It prevents mast cell degranulation and reduces the release of eosinophil cationic protein, a key contributor to ocular surface damage. Additionally, NAAGA inhibits leukotriene B4, a potent chemotactic factor, and blocks complement cascade activation via both classical and alternative pathways. These actions help reduce conjunctival hyperemia, chemosis, and chronic ocular inflammation, offering more comprehensive control of the inflammatory cascade compared to azelastine.
A wide array of studies support NAAGA’s superior efficiency. In a study led by Shin et al., those treated with NAAGA experienced a reduction in OSDI scores from 30.41 ± 20.08 at baseline to 7.74 ± 7.19 at 3 months. However, those on cyclosporine A illustrated a smaller improvement from 25.30 ± 19.04 to 13.63 ± 14.94. TBUT also increased more substantially in the NAAGA group, from 3.28 ± 1.49 to 5.92 ± 2.31 seconds, as opposed to 3.83 ± 2.04 seconds in the cyclosporine A group. Patients reported lower discomfort scores with NAAGA, denoting better tolerability.
Brignole-Baudouin et al. further portrayed that NAAGA markedly reduced the expression of HLA-DR, an indicator of ocular surface inflammation, bolstering its role in modulating immune activity on the ocular surface. Additionally, a preservative-free formulation combining NAAGA with hyaluronic acid and trehalose remarkably improved dry eye symptoms, as reported by El Fekih et al., with a mean OSDI score reduction of 44.6 ± 15.9 points over 42 days. Notably, 96.8% of patients also depicted improvement in conjunctival hyperemia (redness of the conjunctiva).
Despite these advantages, practical differences between the two drugs may impact therapeutic decisions. Azelastine yields fast-acting relief, requires only twice-daily dosing, and is extensively available at a lower cost in generic forms. However, its use is typically limited to 6 weeks without medical supervision. NAAGA, on the other hand, needs 4 daily instillations and is currently available as a branded formulation, which may pose cost-linked challenges. Nevertheless, NAAGA is authorized for long-term usage starting at age 4. It comes in preservative-free single-dose units congruent with contact lens wear, and avoids anticholinergic effects. Its broader anti-inflammatory activity and safety profile make it particularly suitable for those requiring sustained ocular surface protection and inflammation control.
To sum up, both azelastine and NAAGA are promising options for the management of coexisting DED and allergic conjunctivitis. Azelastine remains a practical first-line option for those needing immediate symptom relief and simpler dosing. However, NAAGA may be the preferred choice for long-term management, particularly in patients with chronic inflammation, contact lens use, or intolerance to anticholinergic side effects. The selection of therapy should be individualized, on the basis of the patient’s symptom severity, requisition for rapid relief, lifestyle compatibility, and treatment goals.
Although the findings indicate that NAAGA may offer benefits in alleviating inflammation and enhancing tear film stability, further studies are warranted to confirm NAAGA's dual-action potential and expand its clinical applications in ocular surface diseases. Additional investigations must involve larger, controlled clinical trials and assess the potential of combining NAAGA with adjunctive therapies like artificial tears for a more holistic management strategy. Moreover, evaluating inflammatory mediators in subsequent studies could help clarify the underlying mechanisms through which NAAGA exerts its effects.
Ophthalmology and Therapy
Clinical and Biological Evaluation of NAAGA Versus Azelastine Eye Drops in Patients with Allergic Conjunctivitis and Tear Film Dysfunction: A Randomized Controlled Trial
Mario Troisi et al.
Comments (0)