Categories
Change Password!
Reset Password!


This randomized controlled trial sought to determine the safety and efficiency of electronic nicotine delivery systems (E-cigarettes) for smoking cessation.
E-cigarettes combined with counseling increase tobacco abstinence rates when compared to counseling alone.
This randomized controlled trial sought to determine the safety and efficiency of electronic nicotine delivery systems (E-cigarettes) for smoking cessation.
Adults who smoked at least 5 cigarettes daily and were willing to set a quit date were randomized to either an intervention group or a control group. The intervention included free E-cigarettes with e-liquids, standard smoking-cessation counseling, and optional self-paid nicotine-replacement therapy.
The control group was given counseling in conjunction with a voucher, which could be utilized for any purpose, including nicotine-substitution therapy. The key outcome was continuous, biochemically verified avoidance from tobacco at 6 months. Secondary outcomes encompassed self-reported abstinence from tobacco and all nicotine products, respiratory symptoms, and serious adverse events.
A total of 1246 volunteers were enrolled (622 intervention, 624 control). At 6 months, the intervention group showed higher validated continuous abstinence from smoking when compared to the control group (relative risk, 1.77). Self-reported 7-day abstinence from smoking at 6 months was higher in the intervention group, though abstinence from all nicotine was lower. Furthermore, the intervention group experienced fewer serious adverse events and higher non-serious adverse events when compared to the control group, as shown in Table 1:
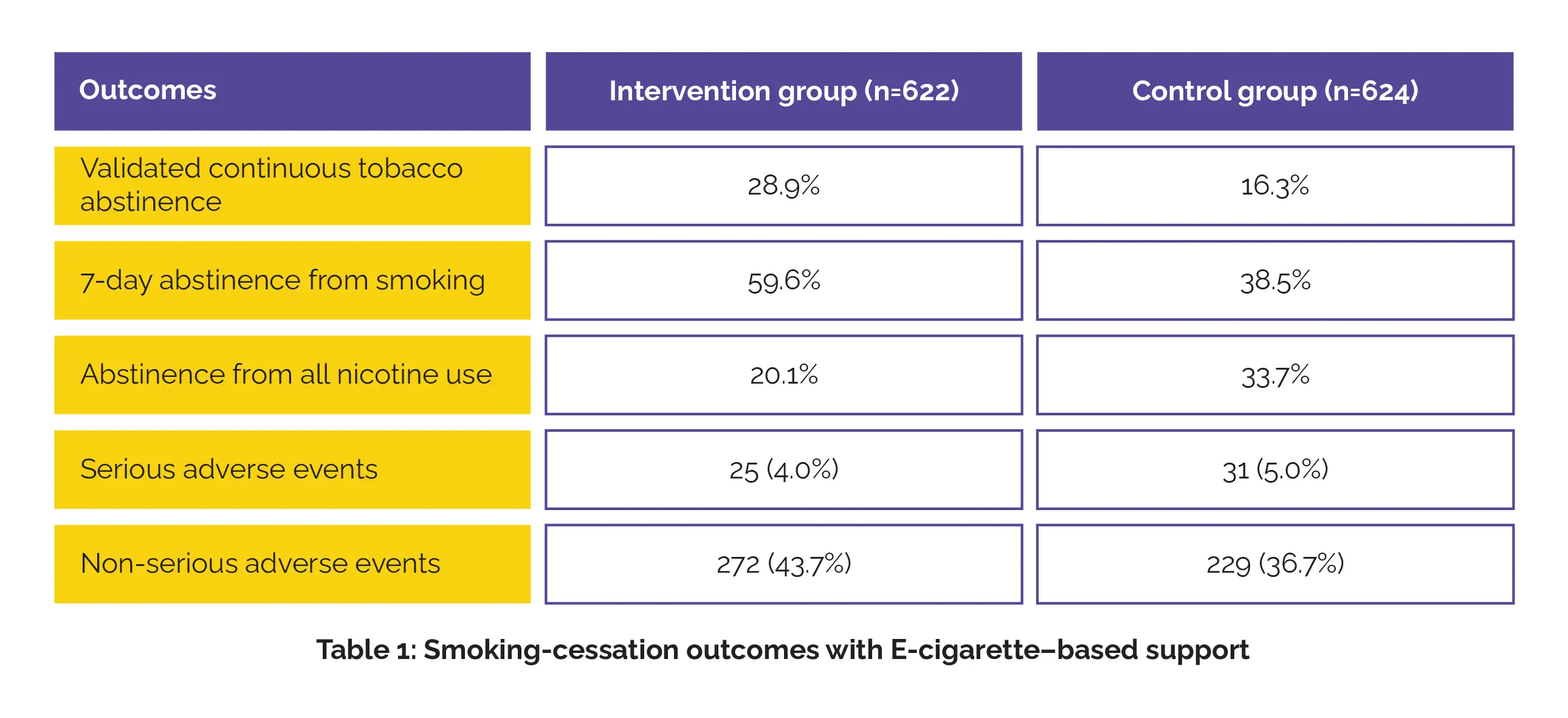
E-cigarettes combined with standard counseling were more effective in achieving tobacco abstinence than counseling alone, without evidence of increased serious adverse events.
The New England Journal of Medicine
Electronic Nicotine-Delivery Systems for Smoking Cessation
Reto Auer et al.
Comments (0)